The AHEAD Study
The AHEAD Study is testing whether an investigational treatment can lower people’s risk of memory loss due to Alzheimer’s disease.

Cutting Edge Research
Researchers have learned that changes in the brains of people with Alzheimer’s disease start as many as 20 years before they notice symptoms like memory problems.
During those two decades, a protein in the brain called “amyloid” builds up in people who go on to have memory problems because of Alzheimer’s disease. While not all people with amyloid in their brain will develop memory problems, we know that the people who do are at a higher risk for developing the disease.
What is the AHEAD Study?
The AHEAD Study is comprised of two different clinical trials testing the same investigational treatment (known as lecanemab) in people who may be at risk for memory problems. Participants will receive a tailored dose of the study treatment, instead of a one-size-fits-all approach. Study participants are enrolled in one of two AHEAD trials based on the level of amyloid in their brain:
- Participants with intermediate amyloid levels take part in the AHEAD A-3 trial—the first ever trial aimed at stopping what may be the earliest signs of Alzheimer’s disease. Participants in the AHEAD A-3 trial will receive lecanemab once every four weeks for four years.
- Participants with elevated amyloid levels take part in the AHEAD A-45 trial. Participants in the AHEAD A-45 trial will receive lecanemab once every two weeks for about two years and then once every four weeks for the remainder of the study.
Participants in both trials will receive intravenous (IV) infusions of lecanemab, tailored to their risk of developing memory loss, or a placebo, an inactive substance designed to mimic the appearance of the investigational treatment. The infusion takes approximately 60 minutes.
At different points in the study, participants have a PET scan (or Positron Emission Tomography brain scan), to look at amyloid and tau (another protein) in their brain. This helps the participants and researchers track their health. The PET scan takes pictures of participants’ brains, allowing researchers to see and track changes in amyloid and tau levels.
Our study is the first of its kind to:
- Enroll participants as young as 55 years old who are at risk of developing symptoms of Alzheimer’s disease as they get older;
- Use a blood test to rule out people not likely to be eligible based on amyloid PET imaging;
- Target the earliest changes in the brain due to Alzheimer’s disease by enrolling participants with intermediate levels of brain amyloid;
- Use an approach that tailors dose levels of the investigational treatment to study participants’ brain amyloid levels;
- Test an investigational treatment that aims to get AHEAD of Alzheimer’s disease.
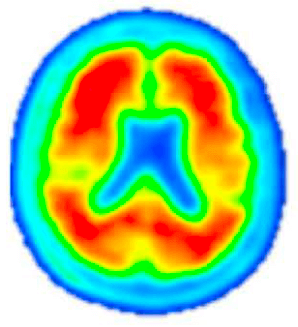
Amyloid PET Scan
Pre-Investigational Treatment
Post-Investigational Treatment
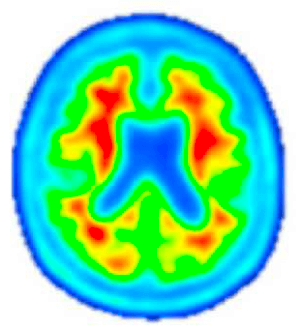
Amyloid PET imaging scans from a representative participant with symptoms of AD in the Phase 2 trial of lecanemab—the investigational treatment being tested in the AHEAD Study. Amyloid PET scans measure the levels of amyloid plaque in the brain. The image on the left is taken before the participant has started on the investigational treatment. The image on the right is taken after 18 months of investigational treatment with lecanemab, indicating a reduction of amyloid plaque burden in the brain. (Data presented at AAIC 2019). The AHEAD Study enrolls participants without symptoms of AD. The effect of lecanemab in participants without symptoms of AD is not known.
About lecanemab:
The investigational treatment being tested in the AHEAD Study
On July 6, 2023, lecanemab was granted full approval by the Food and Drug Administration (FDA) to treat people who already have mild cognitive impairment and mild dementia due to Alzheimer’s disease.
Lecanemab has previously received accelerated approval for its demonstration of reducing amyloid plaque accumulation in the brain in those with mild cognitive impairment and mild dementia due to Alzheimer’s disease.
The AHEAD Study builds on the positive results that lecanemab showed in people with mild cognitive impairment or mild dementia.
This study is testing the effects of lecanemab in people who have no cognitive symptoms of Alzheimer’s disease, but in whom tests indicate amyloid is present in the brain by assessing whether initiating lecanemab in people with amyloid plaques before symptoms start may help prevent cognitive decline. Lecanemab is an investigational treatment in this population.
For participants enrolled in the AHEAD Study:
Participants can receive lecanemab even if they were assigned to placebo, if:
- They complete the study’s full duration, or
- If they experience progression to mild cognitive impairment or dementia during the study and are deemed appropriate for treatment based on the FDA approval of lecanemab
There are stark racial and ethnic differences in Alzheimer’s disease rates. Yet most research trials have not included enough participation of Black or African Americans, Latinos, Asian and Pacific Islanders, and American Indians and Alaska Natives.
The AHEAD Study aims to increase participation from underrepresented groups and needs individuals of every race and ethnicity to help find a treatment for Alzheimer’s disease that works for everyone.
Study Leadership
The study is funded by the National Institutes of Health, in partnership with the pharmaceutical company Eisai. It is being conducted by the NIH-funded Alzheimer’s Clinical Trial Consortium (ACTC), a network of leading academic Alzheimer’s research centers.
The AHEAD Study has approximately 100 study locations worldwide, including in North America, Japan, Singapore, Australia, and Europe—more than 70 of which are in the United States and Canada. The study is led by Alzheimer’s disease research experts and academic leadership at the University of Southern California’s Alzheimer’s Therapeutic Research Institute, Brigham and Women’s Hospital, Massachusetts General Hospital, and Harvard Medical School.
The researchers leading the design and implementation of the study are:

Paul Aisen, M.D.
Director, Alzheimer’s Therapeutic Research Institute

Reisa Sperling, M.D.
Professor of Neurology, Harvard Medical School
Director, Center for Alzheimer Research and Treatment, Brigham and Women’s Hospital and Massachusetts General Hospital

Keith Johnson, M.D.
Professor of Radiology, Harvard Medical School
Director of Molecular Neuroimaging, Massachusetts General Hospital
Neurologist, Brigham and Women’s Hospital

Help Us Get AHEAD of Alzheimer's Disease by Working Closely With World-Renowned Researchers
The AHEAD Study
The AHEAD Study is testing whether an investigational treatment can lower people’s risk of memory loss due to Alzheimer’s disease.

Cutting Edge Research
Researchers have learned that changes in the brains of people with Alzheimer’s disease start as many as 20 years before they notice symptoms like memory problems.
During those two decades, a protein in the brain called “amyloid” builds up in people who go on to have memory problems because of Alzheimer’s disease. While not all people with amyloid in their brain will develop memory problems, we know that the people who do are at a higher risk for developing the disease.
What is the AHEAD Study?
The AHEAD Study is comprised of two different clinical trials testing the same investigational treatment (known as BAN2401 (lecanemab)) in people who may be at risk for memory problems. Participants will receive a tailored dose of the study treatment, instead of a one-size-fits-all approach. Study participants are enrolled in one of two AHEAD trials based on the level of amyloid in their brain:
- Participants with intermediate amyloid levels take part in the AHEAD A-3 trial—the first ever trial aimed at stopping what may be the earliest signs of Alzheimer’s disease. Participants in the AHEAD A-3 trial will receive BAN2401 (lecanemab) once every four weeks for four years.
- Participants with elevated amyloid levels take part in the AHEAD A-45 trial. Participants in the AHEAD A-45 trial will receive BAN2401 (lecanemab) once every two weeks for about two years and then once every four weeks for the remainder of the study.
Participants in both trials will receive intravenous (IV) infusions of BAN2401 (lecanemab), tailored to their risk of developing memory loss, or a placebo, an inactive substance designed to mimic the appearance of the investigational treatment. The infusion takes approximately 60 minutes.
At different points in the study, participants have a PET scan (or Positron Emission Tomography brain scan), to look at amyloid and tau (another protein) in their brain. This helps the participants and researchers track their health. The PET scan takes pictures of participants’ brains, allowing researchers to see and track changes in amyloid and tau levels.
Study Leadership
The study is funded by the National Institutes of Health (NIH), in partnership with the pharmaceutical company Eisai. It is being conducted by the NIH-funded Alzheimer’s Clinical Trial Consortium (ACTC), a network of leading academic Alzheimer’s research centers.
The AHEAD Study has approximately 100 study locations worldwide, including in North America, Japan, Singapore, Australia, and Europe—nearly 75 of which are in the United States and Canada. The study is led by Alzheimer’s disease research experts and academic leadership at the University of Southern California’s Alzheimer’s Therapeutic Research Institute, Brigham and Women’s Hospital, Massachusetts General Hospital, and Harvard Medical School.
The researchers leading the design and implementation of the study are:

Paul Aisen, M.D.
Director, Alzheimer’s Therapeutic Research Institute

Reisa Sperling, M.D.
Professor of Neurology, Harvard Medical School
Director, Center for Alzheimer Research and Treatment, Brigham and Women’s Hospital and Massachusetts General Hospital

Keith Johnson, M.D.
Professor of Radiology, Harvard Medical School
Director of Molecular Neuroimaging, Massachusetts General Hospital
Neurologist, Brigham and Women’s Hospital
